The Elestor Battery Is Proving To Be The Ideal Solution To Reliable And Efficient Energy Storage In The Transition To Green Energy.
The reliance on intermittent, erratic energy sources like the sun and wind is growing as the switch to sustainable energy occurs, while the predictable production of power from fossil fuels is being phased away.
These shifts in energy generation, known in German as the “Dunkelflaute,” cause gloomy and windless periods that can last a few days even though they usually only happen once or twice a year. The issue to be resolved is how to handle these times such that there is always enough electricity available at the most affordable price.
Fuel Cells, Gas-Fired Power Plants, And Electrolysers
One option to close the gap is to use electrolysers to convert excess power produced by the sun and wind during times of overproduction into hydrogen. Gas-fired power plants that use green hydrogen may then turn the hydrogen back into electricity. This is a tried-and-true strategy that makes use of proven technology applied to fossil fuel-based sources. In essence, electrolysers and gas-fired power plants function as a battery or an electrical storage device.
This is a different story economically. These hydrogen-fueled gas-fired power plants will only be in use 10% of the time, according to analyses. Additionally, electrolyzing green electricity to create hydrogen, then combusting that hydrogen to create electricity in a gas-fired power plant, is a very inefficient process. An total energy loss of two thirds is the result of each stage having an energy efficiency of roughly 60%.

This is a different story economically. These hydrogen-fueled gas-fired power plants will only be in use 10% of the time, according to analyses. Additionally, electrolyzing green electricity to create hydrogen, then combusting that hydrogen to create electricity in a gas-fired power plant, is a very inefficient process. An total energy loss of two thirds is the result of each stage having an energy efficiency of roughly 60%.
What Batteries Do
The main issue is how big stores of electrical energy may be realized in the most effective and cost-effective manner in order to address those extended periods of time during which little or no power is generated.
There are many different shapes and sizes of electricity storage devices, including pumped hydro, compressed air, flywheels, super capacitors, electrochemical batteries, and many more. There isn’t a magic technology that can meet all storage needs, but each one has special characteristics that help establish where it belongs in practice.
For example, lithium-ion batteries are the energy density champion, making them the perfect choice for mobile devices, electric automobiles, and perhaps even marine difficulties.
Flywheels are only suited for situations where the brief and quick delivery of peak powers is required or has to be absorbed since they are incredibly rapid but can only produce power for a very short duration.
The fact that the capacity (MWh) and power (MW) of flow batteries are not connected is their most distinctive and distinctive feature. Power and capacity are paired in traditional batteries like lithium-ion or lead acid, thus a high capacity also comes with a high power. Only the battery capacity, not the power, has to be increased to last for longer.
Because of this, flow batteries provide the most cost-effective and long-lasting option, whereas lithium-ion batteries are the preferred technology in applications that just require a short duration of use. In other words, the Elestor battery technology is the marathon runner while the lithium-ion battery is the sprinter.
Elestor Battery As A Two-Way Power Source
In order to find the most cost-effective, 100% carbon-free power source that is produced by the sun and wind while keeping the same level of dependability and availability of electricity, Elestor undertook a thorough investigation two years ago.
The study encompassed all four seasons since it used comprehensive historical data on the production of wind and solar energy over the course of a full year. The predicted relationship between sun and wind has shown to be very positive; hence, when the sun doesn’t shine, the wind frequently blows and vice versa. Therefore, it is crucial to establish the ideal balance between solar and wind energy production.
According to the calculations, a maximum of 130 hours must be covered during the ideal solar and wind conditions, and times this long very occasionally—once or twice a year—occur. Electrical energy must be kept in reserves to cover these lapses in order to maintain the availability of electricity.
The most cost-effective approach to bridge more than 130 hours is using an Elestor battery. Additionally, compared to the 10% described above for electrolysers and gas-fired power plants, the same battery may be utilized virtually continuously for grid balancing in addition to these few times.
The electrolyser and gas-fired power plant combo has a lower overall efficiency than Elestor battery technology. The Elestor system is the superior option both in terms of total capital cost and energy efficiency. The bi-directional power plant of the future may be the Elestor battery, rendering gas-fired power plants unnecessary. A power plant that can generate electricity in both directions can also be used to generate electricity.
In the US, there is already movement toward replacing gas-fired power facilities with batteries. A whole gas-fired power plant on the Moss Landing Project has been replaced by a sizable battery and energy firm, Vistra, which will eventually replace its gas-fired power plants for backup with batteries, totaling 6,000 MW in output.

Combining An Electrolyser And A Fuel Cell Into A Single System
Looking more closely at the Elestor battery’s operation reveals that it functions as both an electrolyser and a fuel cell, earning it the moniker “reversible fuel cell.”
Expenditure Of Capital
The Elestor system combines the functions of an electrolyser and a fuel cell into a single piece of hardware. In contrast to separate electrolysers and gas-fired power plants (or fuel cells), both processes share the same equipment, which results in a significantly reduced upfront cost for the Elestor battery despite performing the same duties.
Efficiency
Another significant distinction is that the Elestor battery’s electrolyser and fuel cell functions employ hydrogen and bromine (Br2) rather than the conventional hydrogen (H2) and oxygen (O2) chemistry. Due to this distinction, the combined energy efficiency of an electrolyser and a gas-fired power plant (or fuel cell) is doubled.
The efficiency of the charging (also known as “electrolysis”) and discharging (also known as “fuel cell”) operations is about 90%. Therefore, the overall chemical efficiency is close to 80%. An overall efficiency of about 70% is achieved by accounting for the so-called parasitic losses for auxiliary systems that all batteries have, such as battery management systems, inverters, sensors, and safety systems. These losses total between 5 and 10%.
The Elestor flow battery is a prime contender to replace gas-fired power plants in the form of bi-directional power plants, according to assessments. Compared to the present system based on conventional electrolysers and hydrogen-fueled gas-fired power plants, it has a substantially lower capital cost, a twice-as-high efficiency, and a nearly continuous utilization.
Innovative solutions are required for a modern energy system. We shouldn’t keep outdated systems running.
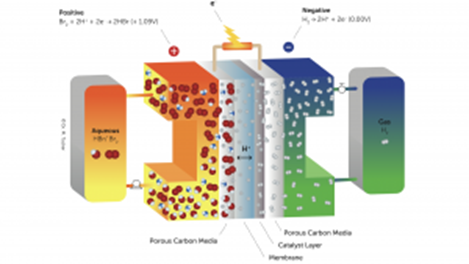
Basics Of Technology
The Elestor battery’s basic operation is seen in the image (below).
A system consists of two tanks, of which the left tank contains hydrogen-bromide (HBr) and the right tank is to store hydrogen (H2). Both tanks are part of an individual closed circuit, which come together at the centre at the so-called membrane. This membrane has a special quality in only permitting hydrogen ions H+ (protons) to flow through.
Electrolyser Charging
The left tank’s HBr molecules are broken up into H+ ions (protons) and BR- ions during charging. Crossing the membrane, the H+ ions pick up one electron to produce hydrogen. The appropriate tank is where this hydrogen is kept. The Br – ions remain in the left circuit, give off electrons, and create Br2. Fundamentally, this charging process is an electrolysis. The Elestor battery divides HBr into H2 and Br2, whereas conventional electrolysis separates H20 into H2 and O2.
Release: Fuel Cell
H2 and Br2 molecules are once again united form HBr during discharge. Two electrons are released by each H2 molecule, creating two H+ ions (protons). In the electrolyte circuit, these protons pass through the membrane and create HBr.
Fundamentally, this discharge procedure is a fuel cell procedure. The recombination of H2 and O2 into H2O occurs in conventional fuel cells, but the Elestor battery recombines H2 and Br2 into HBr.
